
Cryo-Forum
Cryosurgery as a Treatment for Prostate Carcinoma Results and Complications
December 1996
ABSTRACT
BACKGROUND. There has been a resurgence of interest in cryosurgical ablation of the prostate for the treatment of carcinoma. This is due to recent advances in cryosurgical technology, which have resulted in relatively lower morbidity. The objective of this study was to evaluate the effectiveness of ultrasound-guided cryosurgical ablation of prostate carcinoma.
METHODS. Eighty-three patients who had biopsy-proven prostate carcinoma underwent cryosurgical ablation of their entire prostate gland. The initial group of 12 patients had their procedures performed under ultrasound guidance only. The other 71 patients had cryosurgery performed with temperature monitoring in combination with ultrasound guidance. Twelve patients who had positive biopsies underwent a second cryosurgical procedure. All patients had prostate specific antigen (PSA) levels measured at 3, 6, 12, 18, 24, and 30 months after cryosurgery. Ultrasound-guided sextant biopsies were performed at 3-6, 12-18, and 24 months.
RESULTS. The median PSA dropped by 95%, from a preoperative value of 4.3 ng/mL to 0.2 ng/mL 30 months after cryosurgery. The authors experienced a high failure rate (positive biopsies) of 83% for the initial group of 12 patients who did not have temperature monitoring during the cryosurgical procedure. This was in contrast to a success rate of 90% (negative biopsies) for the next 71 patients, who did have temperature monitoring (P < 0.05, chi-square test). Twelve patients underwent a second cryosurgery, and the success rate for this group was 91% (11 of 12 patients). The combined success rate for both the first cryosurgery and the second was 94% (62 of 77 patients). Complications included urethral sloughing, urinary incontinence, impotence, bladder neck contracture, and bladder contracture. The majority of patients recovered rapidly from their cryosurgical procedures and were able to resume normal activities 3-4 weeks afterward.
CONCLUSIONS. These preliminary results demonstrate that cryosurgical ablation of the prostate is a viable treatment option for prostate carcinoma. In the authors' experience, ultrasound alone may not be adequate for monitoring the entire cryosurgical procedure. The authors found that temperature monitoring shortened their learning curve, enabled them to freeze prostate tissue more aggressively, and may have contributed to their overall success.
KEYWORDS: prostate carcinoma, cryosurgery, cryoablation, cancer treatment, prostate neoplasm, temperature monitoring.
C
ryosurgery of the prostate was introduced in the early 1960s.1,2 However, it was quickly abandoned because of the high complication rate, which was due to the inability to control the freezing process and the lack of proper equipment to protect the prostatic urethra.3,4 Recently, there has been a resurgence of this procedure due to refinement of the technique by Onik et al,5,6 who used interventional radiologic procedures and transrectal ultrasound to guide the freezing process. The early experience of Onik et al. with this technique was apparently very promising.6
In order to evaluate the efficacy of this procedure, we have carefully analyzed our experience with 83 patients. Our initial poor experience (positive biopsies) led us to believe that ultrasound alone may be inadequate to guide and control the freezing process. This is due to the critical angle shadowing effect7-9 of the ice ball's casting a shadow that is larger than the ice ball itself. In addition, cell destruction at the rim of the ice ball may not be uniformly complete.10 Because of this, we started to monitor the temperatures of the freezing process by placing thermocouples at the periphery of the gland. We then compared the biopsy results (at 3, 6, 12, and 24 months) between patients who did and did not have temperature monitoring. The results of this analysis are presented herein. We also report our experience with the complications associated with this procedure.
MATERIALS AND METHODS
Between April 1993 and September 1995, 83 patients underwent prostate cryosurgery. The mean age of the patients was 69 years (range, 53-84 years). Because the vast majority of these patients reside in the San Gabriel Valley of southern California, we are continuing to acquire direct follow-up data on 98% of the patients.
All patients who underwent prostate cryosurgery signed informed consent forms approved by the Institutional Review Board (IRB) for human subjects. The IRB-approved consent forms state that the urethral warming device was investigational and that the temperature monitoring system (including the thermocouples) was experimental and not approved by the Food and Drug Administration of the U.S. Department of Health and Human Services (FDA).
All patients had prostate carcinoma diagnosed and staged by transrectal ultrasound-guided biopsies in which the sextant approach was used.11-14 Neurovascular bundles and seminal vesicles were also biopsied in patients with prostate specific antigen (PSA) > 15 ng/mL or in patients who were suspected to have those areas involved with cancer by ultrasound14 or digital rectal exam. As part of the initial staging workup, all patients had a chest X-ray and bone scan. Many of the patients underwent pelvic and endorectal magnetic resonance imaging. Laparoscopic pelvic lymph node dissection (LPND) was recommended for patients with a PSA > 15 ng/mL or with a total Gleason tumor grade > 7.
Androgen Ablation
Because of the inability of 5 cryoprobes to freeze a prostate gland with volume > 45 cc, 47 of the 83 patients were treated with androgen ablation therapy prior to the cryosurgery to shrink the gland. Forty-three patients who had a gland 50-80 cc were treated with leuprolide acetate depot, 7.5 mg intramuscularly every 4 weeks, for an average of 4.5 months (range, 1-15 months). Four patients who had a gland > 80 cc were treated with a combination of leuprolide acetate depot, 7.5 mg intramuscularly every 4 weeks, and flutamide, 250 mg orally 3 times daily for and average of 5.3 months (range, 3-12 months). Two patients were also treated for hot flashes with megestrol acetate, 40 mg orally 3 times daily, for 1 and 4 months.
Transrectal ultrasound was used to recheck the size of the gland after 3 months of therapy. Androgen ablation therapy could be continued for up to 12 months, depending on the response of the gland. All androgen ablation was halted just before or at the time of cryosurgery.
Cryosurgery Procedure
All procedures were performed with the AccuProbe System (Cryomedical Sciences, Inc. [CMSI], Rockville, MD). The Aloka 650 ultrasound scanner (Aloka, Walling Ford, CT), with a biplane probe (transaxial sector 5 MHz and linear 7.5 MHz), was used to guide the cryogenic probe placement and to monitor the freezing process for all patients. All procedures were performed by a two-physician team composed of an urologist (D.C., M.C. or J.C.) and a radiologist (W.W.) experienced in ultrasound.
Initially, we used the fixed-guide technique described by Onik et al.5,6 However, we changed to the free hand placement method described by Lee et al.15 because it allows the freedom of configuring the placement of the
needles and cryogenic probes to the shape of the gland. We used the same mapping technique as Lee et al.15 for probe placement (Fig. 1).
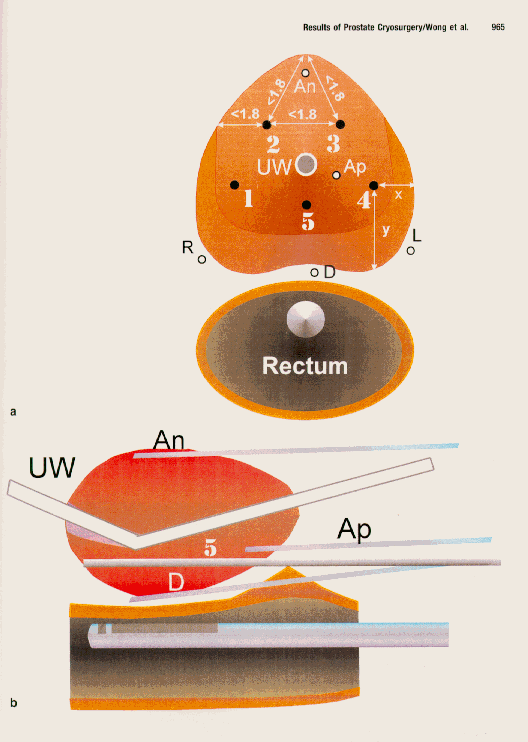
From April to May 1993, our first 12 patients underwent prostate cryosurgery according to the method originally described by Onik et al.5,6 Ultrasound alone was used to monitor the freezing process. The leading edge of the ice ball was originally thought to be fatal to tissue and was delineated on ultrasound as a thin white line (hyperechoic) surrounding an area of acoustic shadowing.5,6 The termination point for the freezing process occurred when the entire prostate gland was encompassed by the area of shadowing and the white line of the ice ball encroached on the muscularis (dark band) around the rectal wall. The cryogenic probes were pulled back by 1-2 cm to ensure that the ice ball encompassed the full length of the prostate gland.
Figure 1
. A planning map for cryoablation is shown. (a) A transverse view, near the mid-gland of the prostate, is shown. Cryogenic probes depicted by are numbered 1-5. Attempts were made to keep the distances between the probes less than 1.8 cm. Probes 1 and 4 are placed in a way that allows distance y to exceed distance x. Thermocouple positions are depicted as open circles. Anterior thermocouple (An) is placed in the anterior fibromuscular stroma. Apex thermocouple (Ap) is imbedded 1 cm within the substance of the prostate in between Probes 4 and 5. The neurovascular thermocouples (R and L) are placed in the posterolateral margins of the mid-gland. (b) A longitudinal view through the midline of the gland is shown. Denonvilliers' thermocouple (D) is placed in between the rectum and the posterior portion of the prostate gland. Probe 5 is placed closer to the prostatic urethra than to the rectum. UW: urethral warming catheter.
Temperature Monitoring
From June 1993 to September 1993, we modified the original technique of Onik et al.5,6 by using thermocouples as part of our procedure on 29 patients. Initially, we placed a thermocouple in Denonvilliers' fascia as an extra safety device to prevent freezing the rectal wall, which would result in urethrorectal fistula. Later, we started placing thermocouples in the region of the neurovascular bundle. Since October 1993, we have consistently placed 5 thermocouples in the following areas (Fig. 1): (1) anterior portion at mid-gland, (2) apex, (3) Denonvilliers' fascia, (4) right neurovascular bundle, and (5) left neurovascular bundle.
Initially, the thermocouples were connected to individual hand-held meters to measure the temperatures. This became quite cumbersome when we used five thermocouples simultaneously. We subsequently developed a computerized data acquisition system that could continuously monitor and record five temperatures simultaneously (Fig. 2). This system graphically displays the temperature on a real-time basis by sampling the five thermocouple temperatures once every second. Temperature records are stored in a data file that can be retrieved for later review.
We currently use a combination of transrectal ultrasound and thermocouple temperature monitoring to determine the end point of the cryosurgical procedure. The target fatal temperature that we try to achieve in all 5 thermocouple positions is below -40 °C.16 Sometimes we cannot achieve the fatal target temperature in certain areas. In such a situation, we hold the freeze longer and utilize another freeze thaw cycle.17 We stop the freezing process prior to achieving our target temperature if the ultrasound image indicates that we may be in danger of freezing the rectal mucosa. Our current technique is to perform a double freeze, a freeze plus a pullback, or a double freeze and then a pullback as determined by gland length, with a target temperature of -40 °C for each freeze cycle.
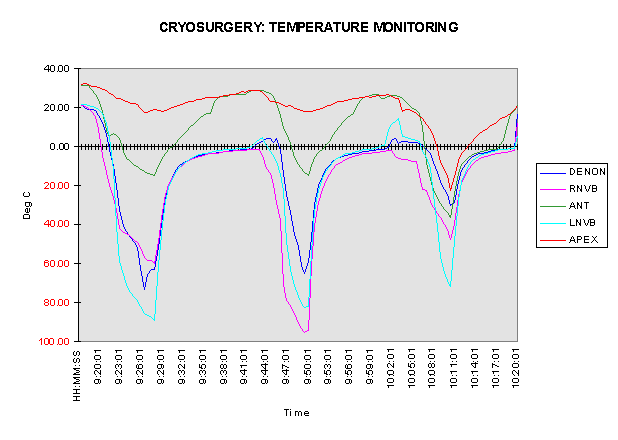
Figure 2
. The computerized data acquisition system used in the study is shown. This chart graphically depicts the temperatures measured by the five thermocouples during the freezing cycles for one patient. This patient underwent three freezes: a double freeze at the base and a pullback to freeze the apex.
Urethral Warmer
We originally used a urethral warming catheter supplied by CMSI to prevent freezing of the prostatic urethra. In April 1994, the FDA removed the warming catheter system from the market. We then used an alternative warming catheter system.18,19 In May 1995, we were able to locate a manufacturer that was able to supply a warming catheter that closely resembled the original design. We also designed our own fluid warmer, which could maintain the water temperature at 44-45 °C.
Follow-Up
All patients were evaluated 3-5 days after surgery. They were then evaluated at 2- to 4-week intervals for approximately 2-4 months. If they had any complications, they were examined at more frequent intervals and over a longer time period.
PSA and Biopsies
PSA levels were drawn at 3, 6, 12, 18, 24, and 30 months after the procedure. Sextant biopsies were performed at 3, 12, and 24 months after surgery. The 3-month biopsies were later changed to 6-month biopsies to allow for further healing after the surgery.
RESULTS
The patients were hospitalized for 1-4 days, with a mean stay of 1.3 days and a median stay of 1 day. Sixty-three of the 83 patients (75%) did not require any form of postoperative pain medication. Most patients were able to return to their normal activities 3-4 weeks after the procedure.
The median Gleason score (combined Gleason tumor grade) for all patients was 5; the majority of the patients had a score < 7. Seventy of the 83 patients had clinical Stage II (TNM system) disease preoperatively, whereas 13 patients had Stage III or higher. Fifteen patients had had previous surgical procedures performed for benign prostate diseases. Seven patients had had previous radiation therapy for prostate carcinoma, but the treatment had failed. The distribution of patients according to Gleason scores, initial clinical stages, and previous therapies is shown in Table 1.
PSA and Biopsy Results
The median PSA levels of the group consistently remained below 0.3 ng/mL after cryosurgery, with a range of 0.1-10.3 ng/mL. The PSA levels of all patients before, at the time of, and after cryosurgery are listed in Table 2.
TABLE 2
Prostate Specific Antigen Levels before, at the Time of, and after Cryosurgery
PSA @ Diagnosis | PSA @ cryosurgery | PSA @ 3 mos | PSA @ 6 mos | PSA @ 24 mos | PSA @ 18 mos | PSA @ 24 mos | PSA @ 30 mos | ||
N | 83 | 79 | 79 | 65 | 55 | 32 | 18 | 16 | |
Mean | 11.2 | 6.7 | 0.4 | 0.5 | 0.7 | 0.7 | 1.2 | 0.3 | |
Median | 7.5 | 4.2 | 0.2 | 0.2 | 0.2 | 0.3 | 0.3 | 0.3 | |
Range | 0.6 - 83.6 | 0.2 - 83.6 | 0.1 -6.2 | 0.1 - 6.1 | 0.2 - 6.9 | 0.1 - 7.9 | 0.1 - 10.3 | 0.1-1.0 | |
Seventy-eight of 83 patients have had follow-up prostate biopsies. At the time of this report, 4 patients were not yet due for their 6-month biopsies. One patient did not have a biopsy because he was on warfarin sodium therapy, and his PSA has remained <0.15 for 2 years. Of the group of patients who did not have temperature monitoring, 10 of 12 (83%) had positive biopsies. In contrast, only 6 of the remaining 66 patients who had temperature monitoring had positive biopsies. The overall positive biopsy rate was 21% (16 of 78 patients). The biopsy results are summarized in Table 3.
TABLE 3
Biopsy Results after Cryosurgery
| 3 to 6 mos | 12 mos | 18 mos | 24 mos | Overall |
No Thermocouples |
|
|
|
|
|
N | 12 | 5 | 1 | 3 | 12 |
Positive | 7 (58%) | 1 (20%) | 1 (100%) | 1 (33%) | 10 (83%) |
Negative | 5 (42%) | 4 (80%) | 0 | 2 (66%) | 2 (17%) |
With Thermocouples |
|
|
|
|
|
N | 64 | 53 | 1 | 22 | 66 |
Positive | 3 (5%) | 3 (6%) | 0 | 1 | 6 (9%) |
Negative | 61(95%) | 50(94%) | 1 (100%) | 21(95%) | 60 (91%) |
Total |
|
|
|
|
|
N | 76 | 58 | 2 | 25 | 78 |
Positive | 10 (13%) | 4 (7%) | 1 (50%) | 2 (8%) | 16 (21%) |
Negative | 66 (87%) | 54 (93%) | 1 (50%) | 23 (92%) | 61 (79%) |
Four patients had negative postoperative biopsies but still had inappropriately high PSA levels. They underwent laparoscopic lymph node dissection. On restaging, 3 of those patients were found to have lymph node metastases. Three of these patients had PSA levels >20 ng/mL, but they refused preoperative staging pelvic lymphadenectomy. The third patient had a PSA level < 15 ng/mL and was not felt to be at high risk for metastatic disease.
Androgen ablation
Forty-seven patients had androgen ablation prior to cryosurgery, and the other 37 patients did not. All androgen ablation was for downsizing only and was discontinued before or at the time of cryosurgery. For 24 months there were no statistical differences in PSA values and biopsy results between those who did have androgen ablation therapy and those who did not (P < 0.000).
Thermocouple monitoring
There was a significant difference in the success of the cryosurgical procedure between the initial group of 12 patients who did not have thermocouple temperature monitoring and the group of 62 patients who did. The success rate of the first group was only 17%, whereas that of the second group was 90% (P < 0.0001, chi-square test).
Eight of the 10 patients in the nonthermocouple group underwent repeat cryosurgery with thermocouple temperature monitoring. The other two patients elected to have androgen ablation therapy and delay their repeat cryosurgery until a later date. The mean PSA value of these 8 patients who had repeat cryosurgery was 0.1 ng/mL, and all their biopsies have been negative for 2 years.
Gleason score and clinical stage
There was no statistically significant relationship identified between Gleason score versus the outcome of the first cryosurgery procedure (P = 0.310, chi-square test). Eighty-three percent of the patients (5 of 6) with Gleason scores > 8 had negative biopsies up to 2 years after the procedure.
Patients who had disease at more advanced clinical stages did nearly as well as those who had less advanced disease. Seventy-five percent of the patients (9 of 12) who had clinical Stage III disease had negative biopsies up to 2 years after the procedure. Eighty-two percent of the patients (56 of 65) who had Stage II disease were successfully treated for 2 years. There was no statistical significance in the overall success rates between these two groups (P = 0.320, chi-square test). It may be that the sample of Stage III disease patients was too small for an accurate comparison. However, we also aggressively froze beyond the prostate capsule in all of our patients.
Previous radiation treatment
Seven patients had had previous external beam radiation treatment for prostate carcinoma. Only one of the seven had a positive biopsy after cryosurgery. There was no significant difference in the success rates of cryotherapy between radiated and nonradiated patients (P = 0.62, chi-square test).
Repeat cryosurgery
Twelve patients had a second cryosurgery because they had positive biopsies. Eleven of the 12 (92%) were successfully retreated, as defined by negative biopsies up to 2 years after the second procedure. Hence, the overall success of the combined first and repeat cryosurgeries was 94% (72 of 77 patients who had biopsies).
Complications
The major complications of cryosurgery included sloughing (necrosis) of the prostatic urethra, bladder neck contracture (BNC), incontinence, and impotence. The first three complications occurred when the warming catheter was not in intimate contact with portions of the prostatic urethra, the bladder neck, or the external sphincter. Tables 4 and 5 demonstrate the complication rates with and without the CMSI warming system and those with and without previous therapy.
TABLE 4
Relationship between Previous Treatments and Complications a
Complications No previous Previous Previous Previous treatment surgery radiation cryosurgery
N 71 8 7 12
Urethral sloughing 27 (38%) 7 (88%)b 6 (86%)b 4 (33%)
Bladder neck 8 (11%) 1 (13%) 4 (57%)b 1 (8%)
contracture
Incontinence
mild 0 1 (13%) 2 (29%) 0
moderate 1 (1%) 0 0 0
severe 2 (3%) 1 (13%)b 4 (57%)b 2 (17%)
N
: no. of patients.a Includes cases with and without the original urethral warmer.
b Indicates statistical significance by Fisher's exact test or chi-square test with P < 0.05.
Urethral slough
Urethral slough, a result of necrosis of the prostatic urethra, is the most common complication after cryosurgery (having occurred in 45 of 95 patients in this study, or 47%). The diagnosis of urethral slough is made cystoscopically, regardless of symptoms. The earliest sign may be asymptomatic pyuria. The slough may be confined to a small portion of the prostatic urethra (mild); occupy a moderate portion, accompanied by symptoms (moderate); or occupy the entire prostatic urethra (severe).
The incidence of sloughing was 38% for patients who had not had previous therapies. In contrast, the incidence was 88% for those who had had previous prostate surgeries and 86% for those who had had previous radiation. These differences were statistically significant (P < 0.05, chi-square test) (Table 4). There was also a significant difference between the incidences of sloughing between those who had the original CMSI warming system (28 of 75 patients, or 37%) and those who did not have the warming system (17 of 20 patients or 85%; P < 0.05, chi-square test) (Table 5).
In general, we treat the mild and some of the moderate sloughs with watchful waiting and catheterization. However, severe sloughs were treated with transurethral prostatectomy (TURP). Of all the patients who had a slough, 44% required a TURP, independent of the warming device and whether or not the patients had had previous surgery (Tables 4 and 5).
TABLE 5
Complications of Cryosurgerya
Complications With original Without original
warmer (n = 75) warmer (n = 20)
Urethral sloughing 28 (37%) 17 (85%)
Bladder neck contracture 3 (4%) 11 (55%)
Incontinence
Mild 2 (3%) 1 (5%)
Moderate 1 (1%) 0 (0%)
Severe 1 (1%) 8 (40%)
n
: no. of patients.a
Includes glands with previous therapy. P = 0.000 for sloughing, bladder neckcontracture, and incontinence by the chi-square test.
Bladder neck contracture
BNC is defined as severe scarring of the bladder neck, which creates outlet obstruction. Scarring of the prostatic urethra can also occur as a result of cryosurgery, but it is included in the same statistical data because they usually occur together (Tables 4 and 5). In this study, this complication occurred only in patients in which the CMSI warming device was not utilized, or in those who had had prior therapy. Patients who had had previous radiation therapy had the highest incidence of BNC (4 of 7, or 57%, P < 0.05, Fisher's exact test).
Incontinence